Not an exposition, but a question. Why is yellow food colouring red?
While making a movie the other day, I purchased some yellow food colouring and some red food colouring. It wasn’t till I got it home that I was astonished to find that the yellow colouring was red! Even the bottle top was red!
Now I can begin to explain this for myself – I am not there yet – but I was surprised to say least. So while I try to understand this, can someone please explain to me ‘Why is yellow food colouring red?’
UPDATE
My colleague Dave Lowe kindly sent me a link to a site which discusses a related problem . The answer has to do with the amount of light transmitted by the food colouring at different wavelengths. And it is also another example of something we touch upon in Protons for Breakfast, that the colour of an object depends three things: the spectrum of light illuminating the object; the way light travels through or is reflected from the object; and the different sensitivity of our eyes.
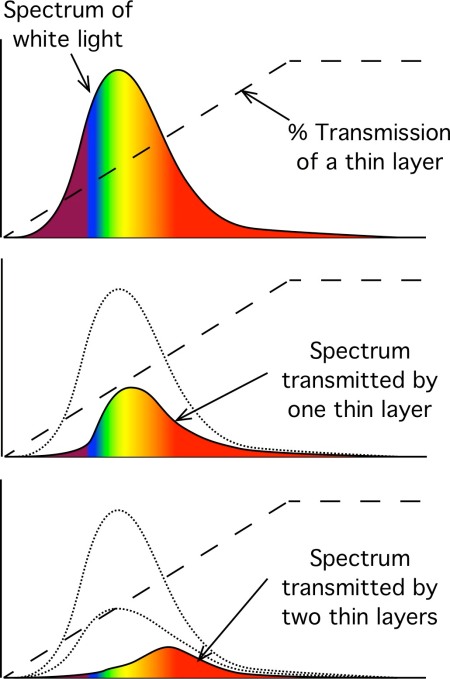
Imagine the spectrum of white light shown above, and imagine that the food colouring molecules absorb all the high frequency light (which we perceive as blue), a middling amount of the middle wavelengths (which we perceive as yellowy-orange), and almost none of the low frequency light (which we perceive as red). Because white light has more of the middle wavelengths present, then even though the food colouring transmits a higher fraction of red light, thin layers or small amounts of the colouring appear yellow. But as the thickness of the food colouring increases, something unusual happens, something described as Beer’s law.
One thin layer: The blue end of the spectrum is strongly absorbed even by a thin layer of the food colouring, so a thin layer transmits almost no blue light. The yellow middle of the spectrum is quite heavily absorbed – let’s guess 50% absorption by a thin layer – but because white light contains lots of yellow light, there us still lots of yellow light being transmitted. The red light is almost all transmitted but there is not much present in white light.
Two thin layer: Almost no blue light made it through the first thin layer – and even less makes it through the second or subsequent layers. Half (50%) of the yellow middle of the spectrum made it through the first thin layer and so 50% of that (1/2 x 1/2 = 1/4) gets through the second layer. Nearly all the red light that was transmitted through the first layer also makes it through the second layer.
More thin layers making a thick layer: Since no blue light made it through the first layers, non will make through a thick layer. For each layer thickness, and additional 50% of the yellow light is absorbed. After 10 thin layers that amounts to (1/2 x 1/2 x 1/2 x 1/2 x 1/2 x 1/2 x 1/2 x 1/2 x 1/2 x 1/2 ) which is approximately one thousandth of the original yellow light. Nearly all the red light that was transmitted through the first layer and the second layers also makes it through the subsequent layers. So after many layers, the initial dominance of the yellow transmitted light is replaced by dominance of the red light light.
Tags: Beer Lambert Law, Beer's Law, Colour, Food Colouring

December 31, 2018 at 2:09 am |
Dude, that reply was too complex. Simplify.