In part 2 I looked at transmission of infrared light through a gas containing a molecule which absorbs infrared light at one particular frequency.
We saw that at higher concentrations, the absorption at specific frequencies broadened until entire bands of frequencies were ‘blocked’.
We saw that the width of the ‘blocked bands’ continued to increase with increasing concentration.
Here we look at how that insight can be applied to transmission of infrared light through Earth’s atmosphere.
This is even more complicated.
- We are mainly interested in transmission of infrared light from the Earth’s surface out through the atmosphere and into space, but the atmosphere is not at a uniform temperature or pressure.
- When absorbing gases are present, the air is not just a ‘conduit’ through which infra-red light passes – the air becomes a source of infrared radiation.
- We are mainly interested in the effect of carbon dioxide – but there are several other infrared ‘active’ gases in the atmosphere.
- Gases are not the only thing in the atmosphere: there is liquid water and particulates.
So it’s complicated: Here are a few more details.
1. Density.
If the carbon dioxide is distributed in a fixed proportion to the amount of oxygen and nitrogen through the atmosphere, then it will have more effect where the atmosphere is most dense: i.e. lower down in the atmosphere.
And density is affected by both temperature and pressure.
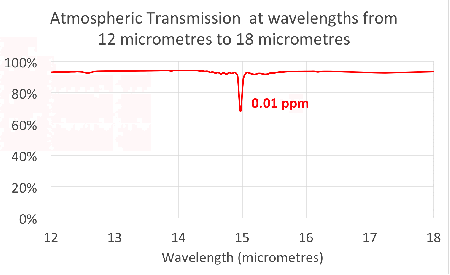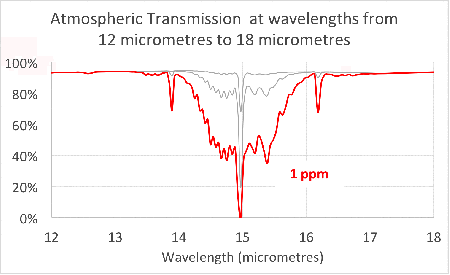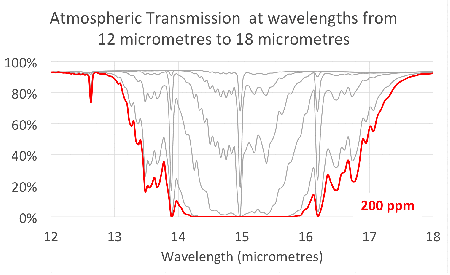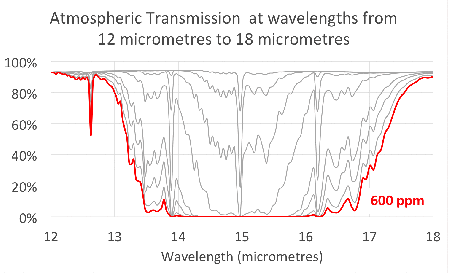
Since carbon dioxide molecules absorb 100% of the infrared light with wavelengths around 15 micrometres, as we saw in the previous article, increasing the concentration of carbon dioxide increases the range of wavelengths that are ‘blocked’. This is illustrated in the figure at the head of the article.
Increasing the concentration of carbon dioxide also changes the height in the atmosphere at which absorption takes place.
2. Re-radiation.
Once absorbed by a carbon dioxide molecule, the infrared light does not just disappear.
It increases the amplitude of vibration of the molecule and when the molecule collides with neighbouring molecules it shares that energy with them, warming the gas around it.
A short while later the molecule can then re-radiate light with the same frequency. However the brightness with which the gas ‘glows’ relates to its local temperature.
Some of this re-radiation is downward – warming the Earth’s surface – and giving rise to a ‘greenhouse’ effect.
And some of this re-radiation is upward – eventually escaping into space and cooling the Earth.
3. Other things.
Carbon dioxide is not only the infrared active gas in the atmosphere. There is also methane, ozone and, very significantly, water vapour.
There is also condensed water – clouds.
And then there are particulates – dust and fine particles.
All of these affect transmission of light through the atmosphere to some extent.
For an accurate calculation – all these effects have to be considered.
MODTRAN
Fortunately, the calculation of transmission through the atmosphere has been honed extensively – most notably by the kind people at the US Air Force.
However the code is available for anyone to calculate atmospheric transmission.
David Archer and the University of Chicago kindly host a particularly friendly front end for the code.

Aside from just clicking around, it is possible to download the results of the calculations and that is how I plotted the graphs at the head of the page.
To get that data I removed all the other greenhouse gases from the atmosphere (including water), and varied only the concentration of carbon dioxide.
Notice that the absorption lines grow into bands that continue to broaden as we add more and more carbon dioxide. This is exactly what we saw in the simple model in the second article.
This shows that the transmission through the atmosphere is still being affected by additional carbon dioxide, and these bands have not ‘saturated’.
Asking a question
MODTRAN can answer some interesting questions.
Assuming that the Earth’s surface is at a temperature of 15 °C, we can ask MODTRAN to calculate how much infrared light leaves the top of the atmosphere (100 km altitude) as we add more carbon dioxide. The result of these calculations are shown below:

The first thing to notice is the qualitative similarity between this graph – the result of complex and realistic calculations – with the simple spreadsheet model I showed in the second article.
The second thing to notice is that the calculations indicate that increasing the concentration of carbon dioxide in the atmosphere reduces the amount of radiation which escapes at the top of the atmosphere. And that it will continue to do so even as the concentration of carbon dioxide increases well beyond its current 400 parts per million (ppm).
Where does that absorbed radiation go? The graph below shows the results of another calculation. It imagines being on the ground and asks how much infrared light is re-radiated back to the Earth’s surface as the concentration of carbon dioxide increases.

The graph shows that matching the decline in infrared radiation leaving the top of the atmosphere, there is a matching increase in radiation falling back down to Earth.
Importantly, both these effects still depend on the concentration of carbon dioxide in the atmosphere even as the concentration grows past 400 ppm.
Over the longer term, this increase in downward radiation will increase the temperature of the Earth’s surface above the assumed 15 °C. This process will continue until the outgoing radiation leaving the top of the atmosphere is balanced with the incoming solar radiation.
That’s all for this article:
In this article we saw that transmission of infrared light through the atmosphere is complicated.
Fortunately MODTRAN software can cope with many of these complexities.
The conclusions of our calculations with MODTRAN are similar to conclusions we came to in the previous article.
Increasing the concentration of a molecule such as carbon dioxide which absorbs at a single frequency will continue to reduce transmission through the atmosphere indefinitely: there is no limit to the amount of absorption.
The next article is about the conclusions we can draw from these calculations.
Tags: Climate Change, CO2, CO2 Bands
January 3, 2017 at 12:58 am |
[…] Michael, why did you write the last four articles (1,2,3,4) on the transmission of infrared radiation through the atmosphere: that stuff is already well […]
January 3, 2017 at 9:27 am |
[…] sense of science « 1. Light transmission through the atmosphere 3. Light transmission through the atmosphere […]
April 20, 2017 at 8:01 pm |
[…] Tom Glazer is describing the basic physics of the MODTRAN model of atmospheric transmission! (Link). […]