In the first article I showed experimental data on the spectrum of light travelling through the atmosphere.
We saw that some frequencies of light are ‘blocked’ from travelling through the atmosphere.
Sometimes this ‘blocking’ occurs at specific frequencies, and sometimes at ranges of frequencies – known as ‘blocked bands’.
In this article, we will consider how both single frequency absorption and blocked bands arise.
Air and Light
Air is composed mainly of nitrogen, oxygen, and argon molecules. The frequencies at which these molecules naturally vibrate are very high, typically greater than 400 terahertz. High frequencies like this correspond to light in the visible or ultraviolet part of the spectrum.
Larger molecules – ones composed of more than two atoms – can vibrate more easily.
They are – in a very rough sense – ‘floppier’ and have lower natural frequencies of vibration, typically a few tens’s of terahertz.
Frequencies in that range correspond to light in the infrared part of the spectrum.
The animation below shows qualitatively the relative frequencies of a vibrational mode of an N2 molecule and a bending mode of a CO2 molecule.
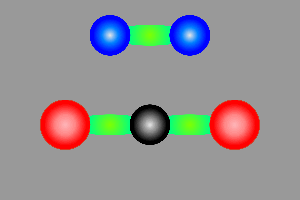
When light travels through a gas containing molecules that can vibrate at the same frequency as the light wave, the molecules begin to vibrate and absorb some of the energy of the light wave.
The molecules then collide with other atoms and molecules and share their energy – warming the gas around them. The light has been absorbed by the gas.
But this absorption only happens close to the specific frequencies at which the molecules vibrate naturally.
The effect of a single frequency of vibration
The figure below shows the effect of the presence of a low concentration of a molecule that can absorb light at a specific frequency.

The figure describes how ‘white’ light – in which all frequencies are present with equal intensity – travels through a non-absorbing gas with a low concentration of molecules which absorb at one specific frequency.
Light with a frequency – represented by a colour: yellow, orange or red – which just matches the vibrational frequency of the molecule is absorbed strongly and doesn’t make it far through the gas.
But light with frequencies on either side of this vibrational frequency is absorbed less strongly. So the percentage of light transmitted has a dip in it at the frequency of molecular vibration.
If we increase the concentration of the absorbing molecule, something really interesting happens.
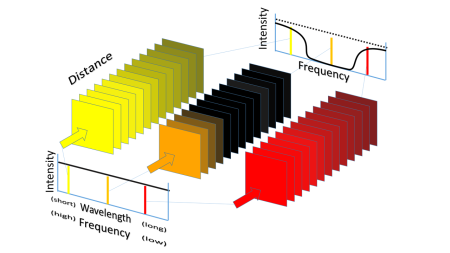
The light at the central vibrational frequency is absorbed even more rapidly. But since it is already 100% absorbed – it doesn’t affect the overall transmission at this frequency. However it does affect where the light is absorbed.
But the additional concentration of absorbing molecules now absorbs strongly on either side of the main absorption frequency.
Eventually, the absorption here becomes so strong that the absorption is 100% even for frequencies that differ significantly from the main vibrational frequency.
This leads eventually to bands of frequencies that are 100% absorbed.
Band Width
Importantly, as the concentration of the absorbing molecule increases – the width of the blocked band increases.
This increase in absorption band width isn’t a property of an individual molecule – each of which just absorbs at frequencies centred around a particular frequency.
The formation of the band – and its width – is a property of a column of gas containing many absorbing molecules
This can be modelled quite easily and the output of a spreadsheet model is animated below as a function of the concentration.
In each frame of the animation, the concentration increases by a factor 2.7 – so that the concentration range covered in the seven frames is 387 (~2.7 to the power 6).

The figure shown in percent on each frame of the animation is the fraction of light in the range from 212 to 228 terahertz which has been absorbed.
Please note that the line-widths and frequencies in the model are arbitrary and approximate. However the qualitative behaviour is universal and independent of the particular mathematics I have used.
- As the concentration of an absorbing gas increases, the transmission at the central absorbing frequency eventually reaches zero.
- As the concentration increases further, the absorption increase at frequencies on either side of the central frequency.
- This eventually forms a range of blocked frequencies – and the width of this blocked range continues to increase with increasing concentration.
The fraction of light transmitted is plotted below.
Once again I would like to emphasise that the graph qualitatively characterises the absorption from a single absorption frequency as a function of concentration.
Significantly, the amount of light transmitted continues to fall even after the transmission at the central frequency reaches zero.
And notice that this broadening of the absorption bands is a property of the transmission of light through a column of gas. It is not caused by line-broadening by individual molecules.
That’s all for this article:
The story so far is that when one looks up through the atmosphere, we see ‘blocked bands’ at a range of frequencies.
In the infrared region of the spectrum, these bands arise from particular modes of vibration of specific molecules which occur at specific frequencies.
In this article we saw that even when the transmission through a gas was saturated, increasing the concentration of the absorbing molecule still reduced transmission through the gas.
This is because the width of the ‘blocked band’ is not a property of the individual absorbing molecules: it arises from transmission of light through a column of gas.
The next article is about how this effect works in Earth’s atmosphere.
Tags: CO2, CO2 Bands, Global Warming
January 3, 2017 at 12:35 am |
[…] Making sense of science « 2: Light transmission through a gas […]
January 3, 2017 at 12:58 am |
[…] Michael, why did you write the last four articles (1,2,3,4) on the transmission of infrared radiation through the atmosphere: that stuff is already well […]
January 3, 2017 at 9:20 am |
[…] sense of science « Still learning after all these years 2: Light transmission through a gas […]